Primary packaging inspection: Vaccinate? But sure!
Not only vaccines must have the highest level of purity and be absolutely safe but also the vials and prefillable syringes from which they are administered. Innovative inspection solutions from HEUFT guarantee this within a short time even with a high output.
Everybody is waiting for a vaccine! In fact for one that can be trusted and has confirmed its effectiveness and tolerability in transparent studies and clinical trials objectively. After all nobody wants to be injected with a vaccine that is not absolutely safe.
When the time finally comes a sterile manufacturing process must also be guaranteed – and last but not least the faultless high-speed filling of extremely large quantities of single doses into perfect primary packaging. Because regardless of whether clear liquid preparations, suspensions which go milky after being shaken up or opaque lyophilisates: full product and packaging safety is essential especially in the case of highly sensitive new vaccines!
Uncontaminated vials
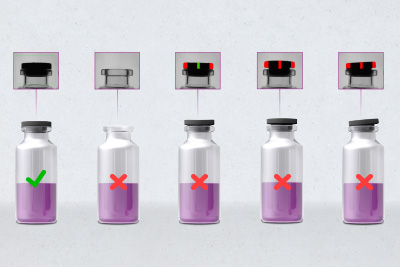
Therefore injection vials from which they are administered parenterally, usually intramuscularly or subcutaneously must be free of cosmetic defects such as dirt, chips and cracks. And they must not contain metal splinters, glass particles, hairs, fibres as well as other foreign matter either which often also transfer fungi or germs. Equally important: full closure integrity so that the functionality of the small vials for a safe injection is always guaranteed and no subsequent contamination can affect the microbial purity of the active pharmaceutical ingredients (API).
This also applies to the oxygen content of the headspace of such a full vial which can trigger oxidation and severely impair the effect of the pharmaceutical product. Therefore it is really good that a module for a laser-based headspace analysis can now also be integrated into the further developed HEUFT spotter II PHS vial inspector. Thus the compact linear machine finds all faults, including the glass particle hidden in the lyo product, which threaten the quality, integrity and safety of such pharmaceutical primary packaging and its contents in one and the same work process together with an innovative procedure for resuspension and special lighting for foreign matter in liquid products which has sunk to the bottom of the vial, sophisticated optical inspection technologies and unique pulsed X-rays.
Error-free prefillable syringes
The risk of an overdose can be specifically avoided by using prefillable syringes in which the vaccine can be packaged as a single dose. For this the practical injection instruments themselves certainly have to meet the highest criteria in terms of purity, integrity and functionality. Manufacturers of prefillable syringes and pharmaceuticals are increasingly relying on the HEUFT Syringer in order to be able to check this.
Since the slim module with new additional optics has been able to make micrometer-sized deformations on the tip of the cannula, so-called needle hooks, visible which can hardly be seen with the naked eye. It has been possible for special glass manufacturers to supply empty disposable syringes which have already been reliably pre-inspected. Standard integrated pulsed X-rays ensure that, in addition, among other things, bent hypodermic needles, pierced protective covers as well as defective luer lock screw adapters and tamper evident closures are already identified before the filling process.
High purity vaccines safely packaged
As always with HEUFT consistent product tracking including rejection verification ensures that every single vial and every single prefillable syringe is inspected and automatically rejected if faulty even during high-speed operation. Whether the HEUFT spotter II PHS or the HEUFT Syringer: both inspection solutions meet fundamental FDA, GMP, GAMP5 and 21 CFR Part 11 requirements reliably. This ensures that, when the time comes, not only the newly launched vaccine itself has the highest level of purity and is safe for the patients but also the primary container in which it is packaged. And that even at high outputs of up to 600 units per minute.